The purpose of this page is to display the list of Serious
Adverse Events for the current Site. Users can click ![]() to add one event at a time or
to add one event at a time or
![]() to edit the desired event in the SAE module.
to edit the desired event in the SAE module.
This tab will be hidden if:
the Enable Serious Adverse Events configuration variable under the Enrollment configuration group is set to False
the current user does not have permission for the SVR Process
The other tabs are Overview, Panels, Monitoring, Narrative, Attachments, Documents, Subjects, Consents, Deviations, Issues, Review, and History.
To view the report as it will print, click
![]() , which opens the report as a PDF file
with the report status as Current
Version Preview and the signature
section is left blank. This button will be hidden if the current
Site Visit Report does
not have a Site Visit Report Template
assigned, or if the Site
Visit Report Status is Approved or
higher. The responses and data fields on the report will represent the
current values at the time the button was clicked, except for any sub-report
sections, which will reflect the current data up to the time the Site
Visit Report Status is changed to First
Draft. This prevents changes in
sub-report data made after the First Draft from inadvertently updating
the Site Visit Report.
, which opens the report as a PDF file
with the report status as Current
Version Preview and the signature
section is left blank. This button will be hidden if the current
Site Visit Report does
not have a Site Visit Report Template
assigned, or if the Site
Visit Report Status is Approved or
higher. The responses and data fields on the report will represent the
current values at the time the button was clicked, except for any sub-report
sections, which will reflect the current data up to the time the Site
Visit Report Status is changed to First
Draft. This prevents changes in
sub-report data made after the First Draft from inadvertently updating
the Site Visit Report.
Click Common Buttons for a description of the buttons found throughout the system and their uses.
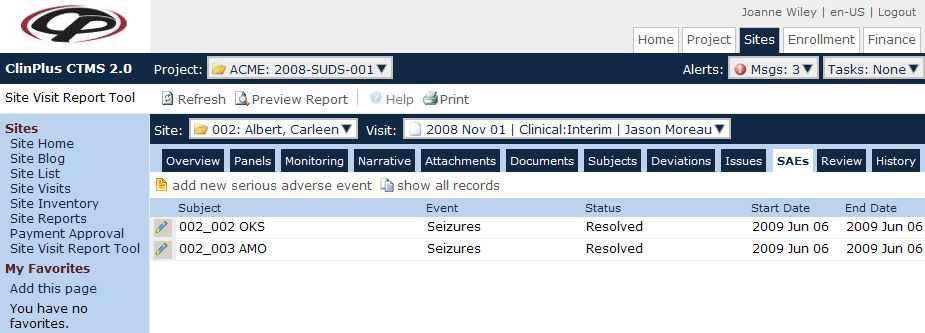
Field |
Description |
Subject |
The number and initials of the patient will be displayed. |
Event |
This column lists the serious adverse event. |
Status |
This column indicates whether the event is Death, Ongoing, Resolved, Unknown, or Other. |
Start Date |
This column lists the date that the serious adverse event started. |
End Date |
This column lists the date that the serious adverse event ended. |