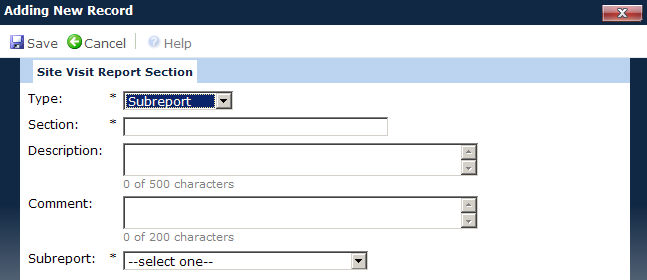
In the Site Visit Report Section module, the user selects whether this section of the report will be either Panel or Sub-report, and then names that portion of the report.
If Panel is selected, then after
the user clicks ![]() ,
the user then goes to the Panels
tab to further define this portion.
,
the user then goes to the Panels
tab to further define this portion.
If Sub-report is selected, the Subreport field displays, and the user selects the report that will be used. A Sub-report section retrieves data from the CTMS database and can include Deviations, Issues, SAEs, Site Documents, Subjects, and custom reports. For these records to appear on the report, the record's date must be on or before the last date of the Site Visit. The report will use the data from the Site Visit Report that was saved at the time the First Draft was created. Click Convert Report to Subreport for steps to create a subreport.
If the section is linked to a template that has been published, the record will be read only.
Click Common Buttons for a description of the buttons found throughout the system and their uses.
Field |
Description |
Type |
The user can select from the following choices:
This field is required when adding a record, otherwise it is read only. |
Section |
The user can enter the name of this part of the report in up to 50 characters. This field is required. |
Description |
The user can enter an explanation of this section in up to 500 characters. |
Comment |
The user can enter a comment with up to 500 characters. |
Sub-report |
This field is only visible when the Type field is set to subreport and then it is required. This field will be limited to the list of Reports with a type of Sub-report and are based on one of the following data sources:
|