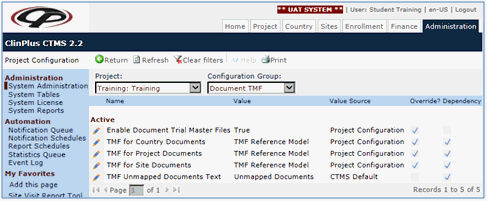
Your ClinPlus CTMS includes the Drug Information Association (DIA) TMF Reference Model Version 2.0 25 June 2012. This Model is not a regulation, but it has become widely accepted as the method to categorize and collect essential documents. Although you cannot edit this model, you can copy and modify it with proper permissions or you can create your own models from scratch.

Click  next to Enable Document Trial Master Files.
next to Enable Document Trial Master Files.
Click the Override field, select True, and click Save.
Override the variables TMF for Country Documents, TMF for Project Documents, and TMF for Site Documents with TMF Reference Model or any of the reference models that you created. You can have different reference models for each of these document types.
Assigning TMF Reference Model to Document Type
Each Document Type needs to be assigned a place within the TMF Reference Model. There are several places within the system that you can map to the reference model.
You can assign a place in the Document TMF Template under either Project Documents, Country Documents, or Site Documents depending on the type of document.
You can assign a place under the Administration tab, System Tables menu item, Document Types, select the base table, select each type of document, click the Trial Master Files tab, and click  next to the appropriate reference model.
next to the appropriate reference model.
You can assign a place when you are uploading the document in either the Project, Country, or Site tabs, under Project Documents, Country Documents, or Site Documents, respectively. Click  next to each type of document, click the Trial Master Files tab, and click
next to each type of document, click the Trial Master Files tab, and click  next to the appropriate reference model.
next to the appropriate reference model.