The Subject Discontinuation Reasons
page displays a list of reasons why a patient may stop their participation
in the study. To add a record, click ![]() or edit a record, click
or edit a record, click ![]() ,
which opens the Subject
Discontinuation Reasons module.
,
which opens the Subject
Discontinuation Reasons module.
Users can filter the records on Active,
Inactive, or All
as well as search for a word or string in the record. To remove all search
options, click the ![]() button.
button.
Click ![]() to return to the Project Tables page.
to return to the Project Tables page.
Columns can be sorted in ascending or descending order by clicking the column heading.
Click Common Buttons for a description of the buttons found throughout the system and their uses.
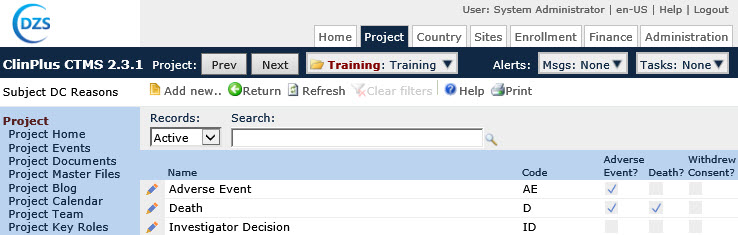
Field |
Description |
Name |
This column lists the names of the categories. |
Code |
This column lists the codes that are used for the categories. |
Adverse Event |
A check in the box indicates that the category is considered an adverse event and is used in calculating the number of subjects who discontinued the study due to an adverse event. |
Death |
A check in the box indicates that the category is considered a death event and is used in calculating the number of subjects who discontinued the study due to death. |
Withdrew Consent |
A check in the box indicates that this category is used to calculate the number of Subjects that discontinued the study due to withdrawal of Informed Consent. |