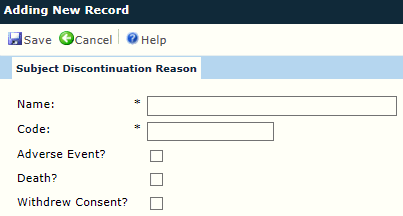
The user can add or edit a single Subject Discontinuation Reason record. After entering the information, click ![]() to return to the Subject Discontinuation Reasons List page.
to return to the Subject Discontinuation Reasons List page.
Click Common Buttons for a description of the buttons found throughout the system and their uses.
|
Field |
Description |
|
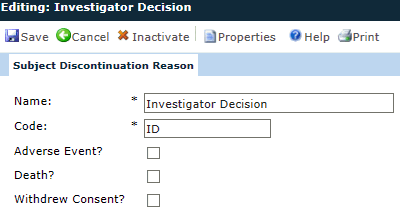
Name |
The user enters the name of the reason why a subject may discontinue in the study using up to 25 characters. This field is required. |
|
Code |
The user enters the code for this reason using up to 10 characters. This field is required. |
|
Adverse Event |
A check in the box indicates that the category is considered an adverse event and is used in calculating the number of subjects who discontinued the study due to an adverse event. |
|
Death |
A check in the box indicates that the category is considered a death event and is used in calculating the number of subjects who discontinued the study due to death. |
|
Withdrew Consent |
A check in the box is used in calculating the number of subjects who discontinued the study due to withdrawal of Informed Consent. |